- Synthetic, latex free, powder free, sterile:E-Beam / Gamma.
- Certificates:CE,MDR,US,FDA,510K,PPE CE,MDSAP
- Color: White Cream .Beaded cuff.
- Design:Anatomical, forward thumb ball, hand Specific.
- Tested high quality exceeds AQL 0.65.
- Micro textured, calibrated grip, medium.
- Shelf Life: 3 Years.
Product Details

MEDISPO® POLYISOPRENE SURGICAL GLOVES FEATURES:
Latex-free: Super soft polyisoprene formulation that is also tough to protect you and patients from pathogens. Exceeds all strength requirements according to USA and EU International standards.
Skin-friendly formulation:Our unique chlorine-free formulation production processes combined with the absence of chemical accelerators known to cause allergic responsesmean skin irritations are minimised or eliminated. Tested according to ISO EN 10993.
Easy donning:Skin friendly polymer coated for easy donning even with damp hands saving you time.
Consistent calibrated grip:The outer surface of Medispo surgical gloves are specially treated to provide a consistent grip. The grip is monitored and controlled during production and each lot must pass stringent internal grip controls before being released.| • Technical data sheet | |||||||||
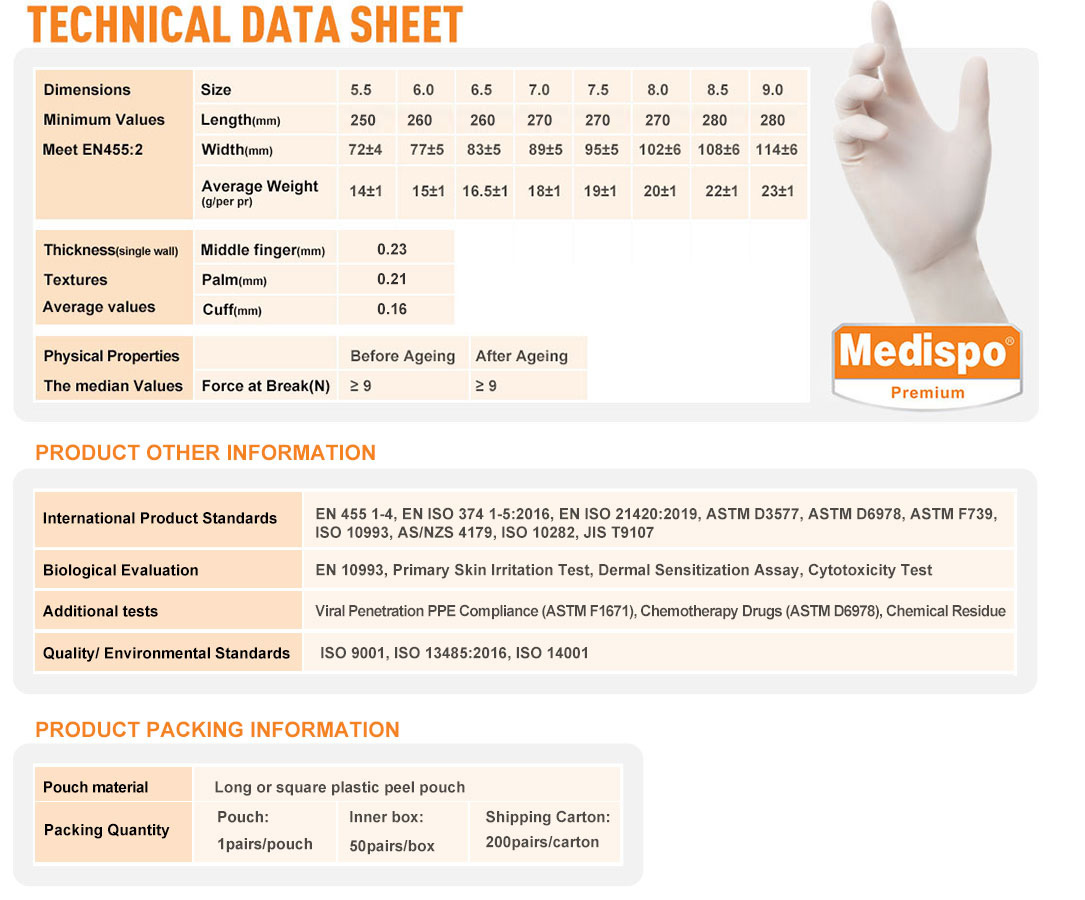
| Dimensions Minimum Values Meet EN455:2 |
Size | 5.5 | 6.0 | 6.5 | 7.0 | 7.5 | 8.0 | 8.5 | 9.0 |
| Length(mm) | 250 | 260 | 260 | 270 | 270 | 270 | 280 | 280 | |
| Width(mm) | 72±4 | 77±5 | 83±5 | 89±5 | 95±5 | 102±5 | 108±5 | 114±5 | |
| Average Weight
(g/per pr) |
16±1 | 17±1 | 18±1 | 19±1 | 20±1 | 21±1 | 22±1 | 23±1 | |
| Thickness (single wall) Textures Average values |
Middle finger(mm) | 0.23 | |||||||
| Palm(mm) | 0.21 | ||||||||
| Cuff(mm) | 0.16 | ||||||||
| Physical Properties The median Values |
Before Ageing | After Ageing | |||||||
| Force at Break(N) | ≥ 9 | ≥ 9 | |||||||
| • Standards & testing certification information | |||||||||
| International Product Standards | EN 455 1-4, EN ISO 374 1-5:2016, EN ISO 21420:2019, ASTM D3577, ASTM D6978, ASTM F739, ISO 10993, AS/NZS 4179, ISO 10282, JIS T9107 | ||||||||
| Biological Evaluation | EN 10993, Primary Skin Irritation Test, Dermal Sensitization Assay, Cytotoxicity Test | ||||||||
| Additional tests | Viral Penetration PPE Compliance (ASTM F1671), Chemotherapy Drugs (ASTM D6978), Chemical Residue | ||||||||
| Quality/ Environmental Standards | ISO 9001, ISO 13485:2016, ISO 14001 | ||||||||
| • Packing information | |||||||||
| Pouch material | Long or square plastic peel pouch | ||||||||
| Packing Quantity | Pouch: 1pairs/pouch |
Inner box: 50pairs/box |
Shipping Carton: 200pairs/carton |
||||||