- Latex, latex free, powder free, sterile:E-Beam / Gamma.
- Certificates:CE,MDR,US,FDA,510K,PPE CE,MDSAP
- Color: White Cream .Beaded cuff.
- Design:Anatomical, forward thumb ball, hand Specific.
- Tested high quality exceeds AQL 0.65.
- Micro textured, calibrated grip, medium.
- Shelf Life: 3 Years.
Product Detail

MEDISPO® ANTIMICROBIAL SURGICAL GLOVES FEATURES:
Special Anti-Virus Technology: Special antiviral technology provides higher quality protection.
Skin-Friendly Formulation: Our Unique Chlorine-Free Formulation Production Processes Combined With The Absence Of Chemical Accelerators Known To Cause Allergic Responses Mean Skin Irritations Are Minimised Or Eliminated. Tested According To ISO EN 10993.
Easy donning:Skin friendly polymer coated for easy donning even with damp hands saving you time.
Consistent calibrated grip:The outer surface of Medispo surgical gloves are specially treated to provide a consistent grip. The grip is monitored and controlled during production and each lot must pass stringent internal grip controls before being released.
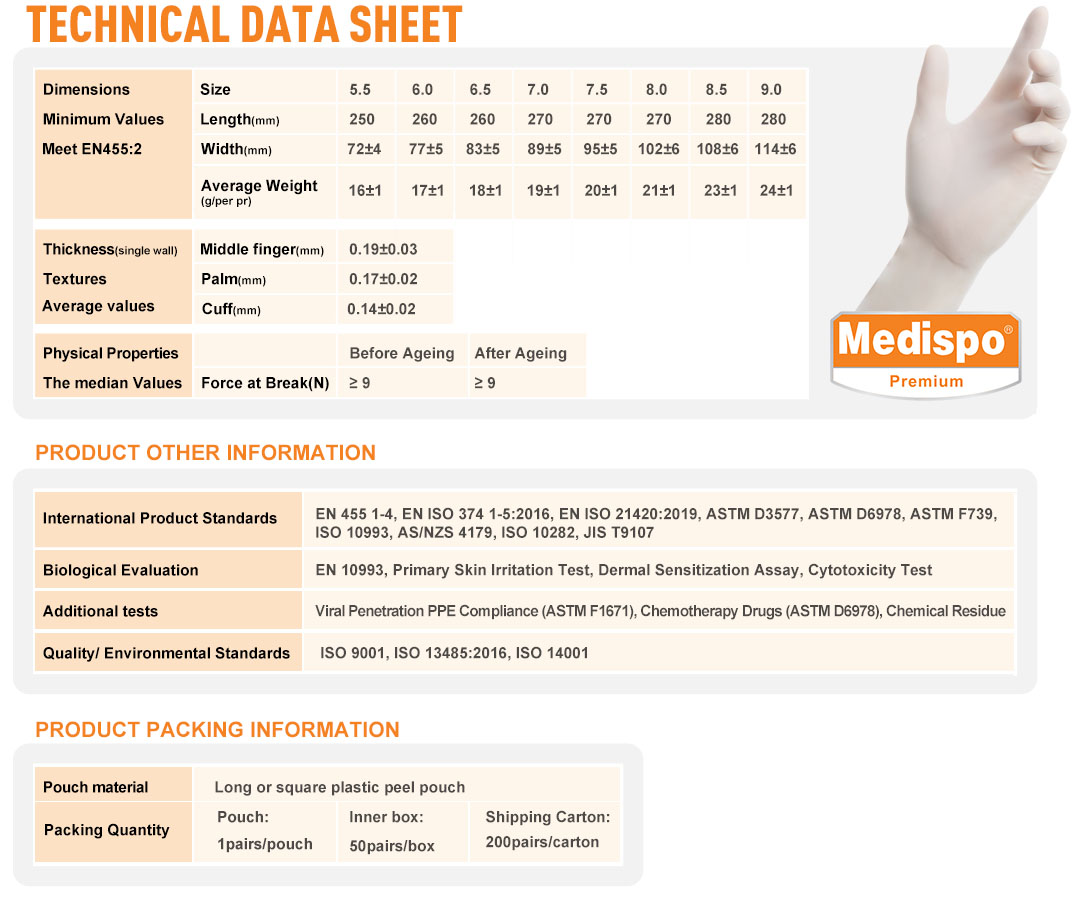
| • Technical data sheet | |||||||||
| Dimensions Minimum Values Meet EN455:2 |
Size | 5.5 | 6.0 | 6.5 | 7.0 | 7.5 | 8.0 | 8.5 | 9.0 |
| Length(mm) | 250 | 260 | 260 | 270 | 270 | 270 | 280 | 280 | |
| Width(mm) | 72±4 | 77±5 | 83±5 | 89±5 | 95±5 | 102±5 | 108±5 | 114±5 | |
| Average Weight (g/per pr) |
16±1 | 17±1 | 18±1 | 19±1 | 20±1 | 21±1 | 23±1 | 24±1 | |
| Thickness (single wall) Textures Average values |
Middle finger(mm) | 0.19±0.03 | |||||||
| Palm(mm) | 0.17±0.02 | ||||||||
| Cuff(mm) | 0.14±0.02 | ||||||||
| Physical Properties The median Values |
Before Ageing | After Ageing | |||||||
| Force at Break(N) | ≥ 9 | ≥ 9 | |||||||
| • Standards & testing certification information | |||||||||
| International Product Standards | EN 455 1-4, EN ISO 374 1-5:2016, EN ISO 21420:2019, ASTM D3577, ASTM D6978, ASTM F739, ISO 10993, AS/NZS 4179, ISO 10282, JIS T9107 | ||||||||
| Biological Evaluation | EN 10993, Primary Skin Irritation Test, Dermal Sensitization Assay, Cytotoxicity Test | ||||||||
| Additional tests | Viral Penetration PPE Compliance (ASTM F1671), Chemotherapy Drugs (ASTM D6978), Chemical Residue | ||||||||
| Quality/ Environmental Standards | ISO 9001, ISO 13485:2016, ISO 14001 | ||||||||
| • Packing information | |||||||||
| Pouch material | Long or square plastic peel pouch | ||||||||
| Packing Quantity | Pouch: 1pairs/pouch |
Inner box: 50pairs/box |
Shipping Carton: 200pairs/carton |
||||||